With increasingly more food products coming onto the market boasting energy boosts with added caffeine, the U.S. Food and Drug Administration (FDA) announced on Monday its intention to investigate the safety of added caffeine and its effect on children and adolescents.
Sparking the investigation was Wrigley’s introduction of a new caffeinated product on Monday. Alert Energy Caffeine Gum contains 40 milligrams of caffeine per piece, marketed as having as much caffeine as a half a cup of coffee.
The Center for Science in the Public Interest (CSPI), a non-profit watchdog group, believes that Wrigley’s website, vividly interactive with strong social media content, is indication that the product will be marketed to young people.
While companies claim to market these products solely to adults, critics say that adding caffeine to items like candy that are attractive to children is still irresponsible. The American Academy of Pediatrics says caffeine has been linked to harmful effects on young people’s developing neurologic and cardiovascular systems.
In a statement, the FDA noted that it was taking a fresh look at the potential impact that “the totality of new and easy sources of caffeine” may have on health, particularly children and younger consumers.
According to Rocky Hill resident Gary Wuerth, 36, this isn’t the first time caffeinated gum has been available to young people. He remembers about 15 years ago when caffeinated gum and the flavorless caffeinated water, Krank2O, was available on military bases. With military personnel working 36-48 hours straight, friends told Wuerth, “This is how we do it.”
“I had some friends in the air force at the time, and they brought me [to the base] to get some,” he said. “The gum was like $1 for a huge pack, and the water was like $5 for a 24-bottle case.”
Both the gum and water “got you jacked up,” he said. “I remember I threw the gum away immediately, and only drank like three of the waters, because there was so much caffeine in them I couldn’t stand it—I thought I was going to have a heart attack.”
Long before the mint and fruit flavors of Alert Energy, Wuerth said the white gum was “incredibly awful tasting,” and was like chewing a tire.
Caffeine is currently an added ingredient to trail mix, potato chips, waffles, and jelly beans. Jelly Belly Extreme Sport Beans contain 50 mg of caffeine per 100-calorie pack, while ARMA Energy is a energy snack line that boasts delivering the same amount of energy as an energy drink, with chips and trail mix. There are Wired Waffles, also with an “energy blend equal to an energy drink,” if not more, which one can serve with caffeinated maple syrup. Frito Lay’s Cracker Jack’D Power Bites are coffee flavored coated wafers that include two tablespoons of ground coffee, approximate 100 mg of caffeine.
“The only time that FDA explicitly approved the added use of caffeine in a food was for cola and that was in the 1950s,” Michael Taylor, FDA deputy commissioner for foods and veterinary medicine, said in a statement on its website on Monday. The current proliferation of caffeine being added to foods is “beyond anything FDA envisioned,” Taylor said.
With the FDA’s investigation, legal authorities are strengthening their assault on energy drinks, for its potential health dangers in young people. According to a 2012 New York Times article, the FDA disclosed that 13 deaths over the previous four years were linked to the consumption of the heavily-caffeinated energy shot, 5-Hour Energy, as well as more than 17 other serious or life-threatening health incidents, such as convulsions and heart attacks. A 2-ounce bottle of 5-Hour Energy is thought to contain approximately 215 mg of caffeine.
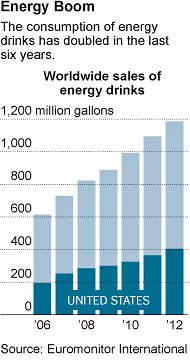 This February, FDA officials said they would take action if they could link the deaths to consumption of the energy drinks, even going so as to force companies to take these products off the market. Companies were forced to stop making alcoholic caffeinated beverages in 2010 because the cocktail of alcohol and caffeine could lead to an alert drunken state resulting in car accidents, assaults and alcohol poisoning.
This February, FDA officials said they would take action if they could link the deaths to consumption of the energy drinks, even going so as to force companies to take these products off the market. Companies were forced to stop making alcoholic caffeinated beverages in 2010 because the cocktail of alcohol and caffeine could lead to an alert drunken state resulting in car accidents, assaults and alcohol poisoning.
In October, the FDA also disclosed that it had received five fatality filings associated with the highly popular energy drink Monster. Last year, Monster Beverage Corp., a Calif.-based company, also was slapped with a subpoena from a state attorney general’s office regarding its ingredient and advertising claims.
On Tuesday, Monster announced it was suing San Francisco City Attorney Dennis Herrera, who asked Monster to provide “adequate warning labels,” cease marketing to minors, and reduce the amount of caffeine in its energy drinks.
 The content of most Monster Energy drinks is approximately 160 mg per 16 oz. can, which is nearly five times the recommended max for adolescents and more than 400 mg per day the FDA has indicated is safe for adults, CBS San Francisco reports. Herrera noted in a letter to the company that Energy Drinks are specifically targeted to youth, who are encouraged to “pound down” drinks in large quantities. Coffee, which may contain more caffeine, are served hot and typically consumed slowly, on the other hand.
The content of most Monster Energy drinks is approximately 160 mg per 16 oz. can, which is nearly five times the recommended max for adolescents and more than 400 mg per day the FDA has indicated is safe for adults, CBS San Francisco reports. Herrera noted in a letter to the company that Energy Drinks are specifically targeted to youth, who are encouraged to “pound down” drinks in large quantities. Coffee, which may contain more caffeine, are served hot and typically consumed slowly, on the other hand.
Energy drinks have more than three times the caffeine of a soda like Coca-Cola. Michael Jacobson, director of CSPI, said that this is problematic because younger people may guzzle energy drinks without realizing how much caffeine they’re consuming.
“Could caffeinated macaroni and cheese or breakfast cereal be next?” said Jacobson. “One serving of any of these foods isn’t likely to harm anyone. The concern is that it will be increasingly easy to consume caffeine throughout the day, sometimes unwittingly, as companies add caffeine to candies, nuts, snacks and other foods.” CSPI wrote the FDA a letter concerned about the number of foods with added caffeine last November.
Taylor said the agency would look at accumulative effect of caffeine on the market. While one product might not cause adverse effects, the rising number of caffeinated products on the market could lead to more adverse health effects for children.


Leave a Reply